Answer:
Qsp > ksp
Step-by-step explanation:
given data
Ag2CrO4 = 9.0 ×
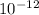
solution
new [
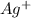 ] =
] =
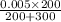 = 0.002 M .................1
= 0.002 M .................1
and
new [
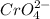 ] =
] =
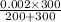 = 0.0012M ..............2
= 0.0012M ..............2
so here solubility equation will be as
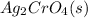 ⇄ 2
⇄ 2
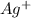 (aq) +
(aq) +
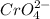 (aq) ......................3
(aq) ......................3
so
Qsp =
![[Ag^+]^2](https://img.qammunity.org/2021/formulas/engineering/college/7g4exkrwb9or124cbcdib256fqm5gyh2ou.png) [
[
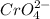 ]
]
put here value
Qsp = (0.002)² × (0.0012)
Qsp = 4.80 ×
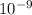
so that we can say that Qsp > ksp