Answer:
a) A Helium nucleus with two protons and one neutron
b) 0.324 of the original amount
Step-by-step explanation:
a) When Tritium decays, the nucleus emits an electron and an antineutrino, which then changes it from a Triton with one proton and two neutrons, to a Helium nucleus with two protons and one neutron.
The decay constant for the decay of Tritium is gotten from
 = 0.693/k
= 0.693/k
where
 is the half life = 12.3 yrs
is the half life = 12.3 yrs
k is the decay constant = ?
substituting, we have
12.3 = 0.693/k
k = 0.693/12.3 = 0.0563
The fraction that will remain after 20 years can be calculated from
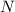 =
=
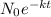
where
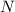 is the final remaining amount of Tritium after 20 years
is the final remaining amount of Tritium after 20 years
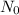 is the original amount of Tritium
is the original amount of Tritium
k is the decay constant = 0.0563
t is the time = 20 years
Equation can be re-written as
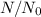 =
=

where
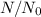 is the fraction of the tritium that will be remaining after 20 years.
is the fraction of the tritium that will be remaining after 20 years.
substituting values, we have
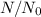 =
=
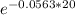
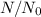 =
=
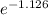
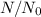 = 0.324 of the original amount
= 0.324 of the original amount