Answer:
The number of grams of sulfuric acid in 1 gallon of battery acid is 1909.4 g.
Step-by-step explanation:
We have:
V = 1 gallon = 3785.4 mL
% m/m = 38.8 %
The number of grams of sulfuric acid can be calculated as follows:
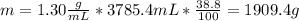
Hence, the number of grams of sulfuric acid in 1 gallon of battery acid is 1909.4 g.
I hope it helps you!