Answer:
The volume at 0.860 atm and 15°C will be 56.638 L
Step-by-step explanation:
Gay Lussac's law explains the variation in the pressure of a gas by modifying its temperature, keeping the volume constant: it determines that pressure and temperature are directly proportional quantities. In other words, Gay-Lussac's law states that when a gas undergoes a constant volume transformation, the ratio of the pressure exerted by the temperature of the gas remains constant:
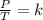
On the other hand, Boyle's law determines that the pressure exerted by a gas is inversely proportional to its volume at constant temperature. This is expressed mathematically as:
P*V=k
Finally, Charles's law says that at constant pressure, the volume of an ideal gas is directly proportional to its absolute temperature and is expressed mathematically as:
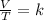
Combined law equation is the combination of three gas laws called Boyle's, Charles's and Gay-Lussac's law:
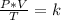
When you want to study two different states, an initial one and a final one of a gas, you use:
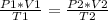
In this case:
- P1= 1.05 atm
- V1= 48 L
- T1= 25 C= 298 K (being 0 C= 273 K)
- P2= 0.86 atm
- V2= ?
- T2= 15 C= 288 K
Replacing:
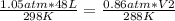
and solving:
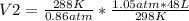
you get:
V2= 56.638 L
The volume at 0.860 atm and 15°C will be 56.638 L