Answer:
2 lone pairs of electrons
Step-by-step explanation:
In this case, the central atom is oxygen. If we check the periodic table we will have an atomic number of 8. So, the electronic configuration would be:
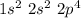
We have 6 electrons in the last level. Therefore, we have 6 valence electrons. For the hydrogens, we will have only 1 electron each. So, in total, we have 8 electrons.
With this in mind, we have to draw two lone pairs in the oxygen and 1 bond with each hydrogen.
See figure 1.
I hope it helps!