Answer:
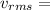 273m/s
273m/s
Explanation: Root Mean Square Speed of an atom or molecule is the speed of a particle in a gas. It is the average speed a particle in a gas can have.
It can be calculated:
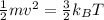
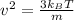
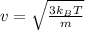
m is mass of one atom or molecule in kg.
An atom of Arsenic sublimes at 614°C. Converting to Kelvin:
T = 614 + 273 = 887K
Molecular mass of As4 is approximately 0.3kg.
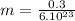
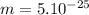 kg
kg
Calculating Root mean square speed :
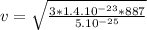
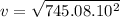
v = 273m/s
The root mean square speed of As4 is approximately 273m/s.