Answer:
Q = 80000 cal
Step-by-step explanation:
La expresin para el calor es
Q = m
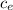 ce ΔT
ce ΔT
el calor especifico del agua es ce= 1 cal/gr ºC
la temperatura de ebullición del agua es Ef = 100ºC y partimos de la temperatura ambiente To= 20 OC
Q = m 1 ( 100 – 20)
q= 80 m
en el ejercicio no se da la masa de agua, pro podemos suponer, pote lleva 1 litros = 100 G
Q = 80 1000
Q = 80000 cal