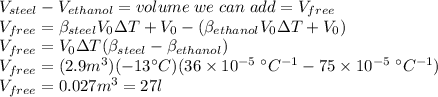Answer:
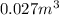
Step-by-step explanation:
First, we write down what we know:
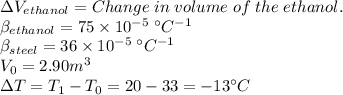
In order to solve this problem, we have to calculate the final volumes of the ethanol and steel respectively and then find their difference, which will give us the volume of ethanol that we can add once the container in cooled.
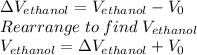
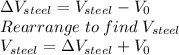
Which gives: