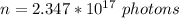Answer:
The value is
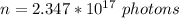
Step-by-step explanation:
From the question we are told that
The amount of power delivered is
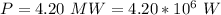
The time taken is
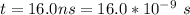
The wavelength is
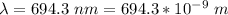
Generally the energy delivered is mathematically represented as
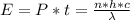
Where
 is the Planck's constant with value
is the Planck's constant with value
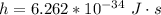
c is the speed of light with value
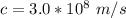
So
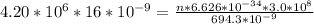
=>