Answer:
0.119 moles
Step-by-step explanation:
Given that,
Mass measured by Nurse Antonio is 7 grams of NaCl
To find,
The no of moles of NaCl
Solution,
The number of moles is given by
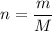
m is given mass
M is molar mass
For NaCl, molar mass is 23+35.5 = 58.5 grams
So,
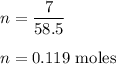
Therefore, there are 0.119 moles of NaCl.