Answer:
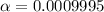
Step-by-step explanation:
Hello,
In this case, given the pH of the weak acid HA, we can obtain the concentration of hydrogen in the solution as shown below:
![pH=-log([H^+]})](https://img.qammunity.org/2021/formulas/chemistry/college/yaheht70p6awnjpu6u9rzcfpwaeb1w7r3o.png)
![[H^+]=10^(-pH)=10^(-4.0)=1x10^(-4)M](https://img.qammunity.org/2021/formulas/chemistry/college/20nghezcidqtq4p3vllh59g2qvywgpm0ld.png)
In such a way, the degree of ionization (
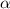 ) is computed as:
) is computed as:
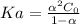
Whereas
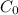 is 0.010 M and the acid dissociation constant is:
is 0.010 M and the acid dissociation constant is:
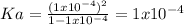
Thus, solving for
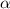 , we obtain:
, we obtain:
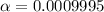
Regards.