Answer:

Step-by-step explanation:
Chemical formulas for every compound:
Potassium vapor =>
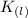
Molten soldium chloride =>
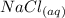
Molten Potassium chloride =>
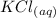
Molten Sodium Metal =>
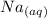
The reaction will be as follows:
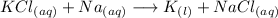
This reaction is usually called displacement reaction in which an element displaces another element of a compund.
![\rule[225]{225}{2}](https://img.qammunity.org/2023/formulas/english/college/eq413d752mwtrwwenrzwldxt4w1olmf1b3.png)