Answer:
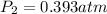
Step-by-step explanation:
Hello,
In this case, we can use the combined ideal gas law in order to analyze its behavior as a function of changing temperature, volume and pressure:
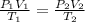
Thus, since we know the volume, temperature and pressure at the initial condition, we can compute the final pressure as shown below:
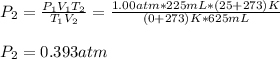
Regards.