Answer: Thus X is Plutonium
Step-by-step explanation:
Alpha Decay: In this process, a heavier nuclei decays into lighter nuclei by releasing alpha particle. The mass number is reduced by 4 units and atomic number is reduced by 2 units.
General representation of alpha decay :
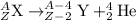
where Z = atomic number
A= mass number
X and Y = atomic symbol of elements
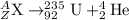
Thus
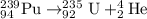
Thus X is Plutonium with atomic number 94 and mass number 239