Answer:
The product would have more acidity than Diethyl malonate
Step-by-step explanation:
For this question, first, we have to start with the structure of the bromination reaction. The bromination would add a "Br" atom in the middle carbon between the ester groups. Therefore, the molecule produced would be diethyl 2-bromomalonate and the formula of this compound fits with the reported by the question:
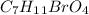
Now, if we have to analyze the acidity we have to check the most acidic hydrogen. In this case, is the "H" in the middle carbon (red hydrogen). In the Diethyl malonate, we have an inductive effect caused by the carbonyl groups on each side of the middle carbon. In the diethyl 2-bromomalonate, we have this same inductive effect plus the Br atom bonded to the same carbon. Therefore, would be easier to remove the hydrogen. So, diethyl 2-bromomalonate would ba more acidity than Diethyl malonate.
See figure 1
I hope it helps!