Answer:
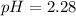
Step-by-step explanation:
Hello,
In this case, for the acid dissociation of formic acid (HCOOH) we have:
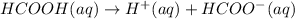
Whose equilibrium expression is:
![Ka=([H^+][HCOO^-])/([HCOOH])](https://img.qammunity.org/2021/formulas/chemistry/middle-school/e95j997enbqrm0s6wlqln8cs3mymr0w68c.png)
That in terms of the reaction extent is:
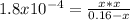
Thus, solving for
 which is also equal to the concentration of hydrogen ions we obtain:
which is also equal to the concentration of hydrogen ions we obtain:
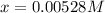
![[H^+]=0.00528M](https://img.qammunity.org/2021/formulas/chemistry/college/ln2mmeje9qsmfn4bpif04si871vbqirwh0.png)
Then, as the pH is computed as:
![pH=-log([H^+])](https://img.qammunity.org/2021/formulas/chemistry/college/80k0jnij8v7qd0dtehezpuvp9exa33y466.png)
The pH turns out:
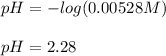
Regards.