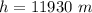Answer:
The value is
Step-by-step explanation:
From the question we are told that
The temperature is
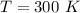
Generally the root mean square speed of the oxygen molecules is mathematically represented as
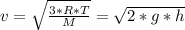
Here R is the gas constant with a value
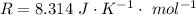
M is the molar mass of oxygen molecule with value
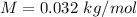
So
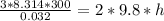
=>