Answer:
B. 980 joules
Step-by-step explanation:
Given the following data
initial temperature T1= 150 °C
final temperature T2= 250 °C
specific heat of gold c= 0.13 J/g°C
mass of gold m= 75.0 grams
we can use the expression stated below to solve for the quantity of heat

Substituting our known data into the expression we can solve for the value of Q
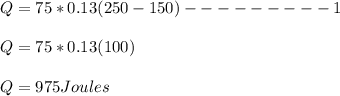
The quantity of heat need to raise the temperature from 150°C to 250°C is 975 J