Answer:
the number of milliliters of a 1M is 402mL
Step-by-step explanation:
The computation of the number of milliliters could be determined by using the following formula
As we know that
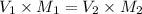
where,
V_1 and V_2 are the starting and final volumes
And, the M_1 and M_2 are the starting and the final molarities
Now the V_1 is
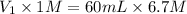
So, the V_1 is 402mL
Hence, the number of milliliters of a 1M is 402mL