Answer:
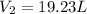
Step-by-step explanation:
Hello,
In this case, by using the Avogadro's law which allows us to understand the volume-moles behavior as a directly proportional relationship:
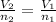
We can compute the volume of 34.3 g of argon by representing it in mole as shown below:
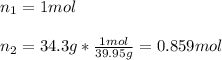
Thus, we find:
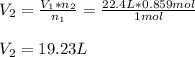
Best regards.