Answer:
At 336.6K, the density of the liquid is 1.212g/cm³
Step-by-step explanation:
The density change in a substance with the temperature. As general (But not always applicable) rule, the density decreases with the temperature.
A formula to find density of the liquid of the problem is:
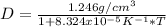
Where D is density and T is absolute temperature (In K).
If we want a density of 1.212g/cm^3:
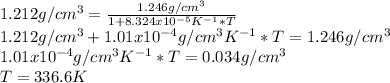
At 336.6K, the density of the liquid is 1.212g/cm³