The question is incomplete. Here is the complete question.
Consider the selenium atom, Se, and the bromine atom, Br.
Write out the full electron configuration fro each atom.
Se:
Br:
Calculate the Effective Nuclear Charge for each atom. Show all of your work for full credit.
Se:
Br:
Which atom is larger?
Answer and Explanation: Electron Configuration of an atom demonstrates the shape and energy of its electrons. One of the rules used for it is given by using Madelung's Rule, in which the order of increased energy of the electrons is:
1s < 2s < 2p < 3s < 3p < 4s < 4p < 5s < 6s < 4f < 5d < 6p < 7s < 5f < 6d < 7p
Atom of Selenium (Se) has 34 electrons. Its electron configuration is
Se:
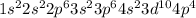
Atom of Bromine has 35 electrons. Its electron configuration is
Br:
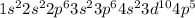
Effective Nuclear Charge (
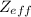 )is the net positive charge experienced by an electron in a multi-electron atom. In other words, it is the net force that helds nucleus and electrons together.
)is the net positive charge experienced by an electron in a multi-electron atom. In other words, it is the net force that helds nucleus and electrons together.
It is calculated by:
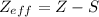
where
Z is the atomic number, i.e., number of protons of the atom
S is the nonvalence electrons, i.e., the number of electrons between the nucleus and the electron in question.
For Selenium (Se):
From the electron configuration, the valence shell is 4 with 6 valence electrons. Nonvalence electrons is the difference between valence and total electrons:
S = 34 - 6 = 28
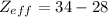 = 6
= 6
The Effective Nuclear Charge of Se is 6
For Bromine (Br):
The valence shell, according to the configuration, is 4 and valence electrons are 7.
S = 35 - 7 = 28
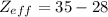 = 7
= 7
The Effective Nuclear Charge fo Br is 7.
Bromine is larger than Selenium because it has bigger Effective Nuclear Charge, which means it held its electrons more loosely and, consequently, has a larger atomic radius.