Answer:
ΔS = - k ln (3)
Step-by-step explanation:
Using the Boltzmann's expression of entropy, we have;
S = k ln Ω
Where;
S = Entropy
Ω = Multiplicity
From the question, the configuration of the molecules in a gas changes so that the multiplicity is reduced to one-third its previous value. This also causes a change in the entropy of the gas as follows;
ΔS = k ln (ΔΩ)
ΔS = kln(Ω₂) - kln(Ω₁)
ΔS = kln(Ω₂ / Ω₁) -------------(i)
Where;
Ω₂ = Final/Current value of the multiplicity
Ω₁ = Initial/Previous value of the multiplicity
Ω₂ =
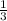 Ω₁ [since the multiplicity is reduced to one-third of the previous value]
Ω₁ [since the multiplicity is reduced to one-third of the previous value]
Substitute these values into equation (i) as follows;
ΔS = k ln (
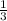 Ω₁ / Ω₁)
Ω₁ / Ω₁)
ΔS = k ln (
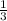 )
)
ΔS = k ln (3⁻¹)
ΔS = - k ln (3)
Therefore, the entropy changes by - k ln (3)