Answer:
The work done by the gas is 55.76 L*atm.
Step-by-step explanation:
The work can be found as follows:
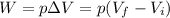 (1)
(1)
Where:
p: is the pressure = 3.4 atm
V(f): is the final volume = 24 L
V(i): is the initial volume = 7.6 L
By replacing the above values into equation (1) we have:
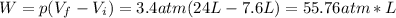
Therefore, the work done by the gas is 55.76 L*atm.
I hope it helps you!