Answer:
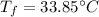
Step-by-step explanation:
Hello,
In this case, we can write the following relationship, explaining that the lost by the hot water is gained by the cold water:
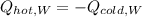
Which in terms of mass, specific heat and temperatures, we have:
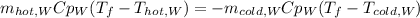
Whereas the specific heat of water is cancelled out to obtain the following temperature, considering that the density of water is 1 kg/L:

Regards.