Answer:
The standard cell potential for this galvanic cell is 0.27 V.
Step-by-step explanation:
The standard redox potentials, E° of the Pb and Cd are:
Pb²⁺(aq) + 2e⁻ → Pb E° = -0.13 V
Cd²⁺(aq) + 2e⁻ → Cd E° = -0.40 V
The standard cell potential for this galvanic cell can be calculated as follows:
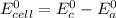 (1)
(1)
Where:
c: is for cathode
a: is for anode
As we can see in the standard redox potentials of Pb and Cd, the Pb is going to be reduced (cathode) and the Cd is going to be oxidated (anode).
By replacing the standard redox potentials of Pb and Cd into equation (1) we have:
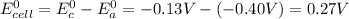
Therefore, the standard cell potential for this galvanic cell is 0.27 V.
I hope it helps you!