Answer:
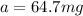
Step-by-step explanation:
Hello,
In this case, we need to remember that for the required time for a radioactive nuclide as radium-226 to decrease to one half its initial amount we are talking about its half-life. Furthermore, the amount of remaining radioactive material as a function of the half-lives is computed as follows:
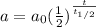
Therefore, for an initial amount of 100 mg with a half-life of 1590 years, after 1000 years, we have:
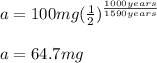
Best regards.