Step-by-step explanation:
We need to find the quantum state of a particle can be specified by giving a complete set of quantum numbers (n,l, ml,ms).
We have the principal quantum number, n = 3
The value of l = n-1
l = 0,1,2,3
The value of
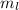
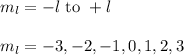
The value of
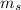
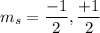
Hence, this is the required solution.