Answer:
E = 0.2276 10⁻¹⁹ J , λ = 8.73374 10⁻⁴ cm
Step-by-step explanation:
Bohr's atomic model can be used for hydrogen-type atoms, that is, they have a single electron in their last orbit, this is the case of doubly ionized Titanium.
It is much easier to work in EV units.
r = a₀ / Z n²
E = - 13.606 (Z² / n²)
where ao is the Bohr radius, which is the ground state orbital of hydrogen a₀ = 0.0529 nm, Z is the atomic number of titanium, and n is an integer that represents the different states of the system.
a) Let's look for the energy, for this we look for the integer numbers of these orbits
r = 13.25 A = 1.325 nm
r = a₀ / Z n²
n = √(Z r / a₀)
the atomic number of titanium is Z = 22
n = √ (22 1.325 / 0.0529)
n = 23
r = 2.12 A = 0.212 nm
n = √ (22 0.212 / 0.0529)
n = 9
now we can calculate the energy of the transition
E = 13.606 (1 /
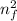 - 1 /
- 1 /
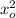 )
)
E = 13.606 (1/9² - 1/23²)
E = 13.6060 (0.010455)
E = 0.1423 eV
Let's reduce to J
E = 0.1423 eV (1.6 10⁻¹⁹ J / 1eV) = 0.2276 10⁻¹⁹ J
To find the wavelength of the transition, use the Planck relation
E = h f
the relationship of frequency and speed of light
c = λ f
we substitute
E = h c /λ
λ = hc / E
λ = 6.626 10⁻³⁴ 3 10⁸ / 0.2276 10⁻¹⁹
λ = 8.73374 10⁻⁶ m
λ = 8.73374 10⁻⁴ cm