Answer:
the solubility of BaCO₃ in pure water and in a solution is 4.472 × 10⁻⁵ M and 6.9204 × 10⁻⁹ M respectively.
Step-by-step explanation:
To calculate the solubility of BaCO₃ in:
(a) pure water and (b) in a solution in which [CO₃²⁻] = 0.289 M
The
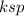 (i.e solubility constant ) for BaCO₃= 2.0 × 10⁻⁹
(i.e solubility constant ) for BaCO₃= 2.0 × 10⁻⁹
BaCO₃ → Ba²⁺ + CO₃²⁻
ksp = s × s
s² = ksp
s =
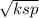
s =

s = 4.472 × 10⁻⁵ M
(b) The solubility of BaCO₃ in a solution in which [CO₃²⁻] = 0.289 M
BaCO₃ → Ba²⁺ + CO₃²⁻
ksp = s × s
2.0 × 10⁻⁹ = s × 0.289
s = 2.0 × 10⁻⁹/0.289
s = 6.9204 × 10⁻⁹ M
Thus, the solubility of BaCO₃ in pure water and in a solution is 4.472 × 10⁻⁵ M and 6.9204 × 10⁻⁹ M respectively.