Answer:
≈ 0.104 liters
Step-by-step explanation:
We can use Boyle's Law:
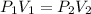
P₁ = 2.50 atm
V₁ = 25.0 mL
P₂ = 457 mmHg
V₂ = ?
Because pressure should be in atm we will convert 457 mmHg to units in atm:
1 atm = 760mmHg so we can divide 457 by 760 and we get ≈ 0.601atm
Next we can plug in the units to the equation for Boyle's Law:
(2.50)(25.0) = (0.601)(V₂)
Solve for V₂
V₂ will give you ≈ 104mL or 0.104L
Either is correct depending on which unit they are asking you to use