Answer:
Silver
Explanation:
Find the volume of the coin
Volume of a cylinder
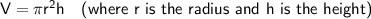
Given:
Substituting given values into the formula to find the volume:
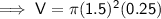
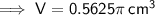
Find the density of the coin given it has a measured mass of 18.54 g
Density formula
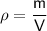
where:
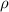 = density
= density- m = mass
- V = volume
Given:
- m = 18.54 g
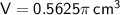
Substituting given values into the density formula:
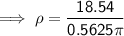
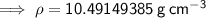
Given:
Therefore, as
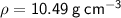 the coin is made from silver.
the coin is made from silver.