Answer and Explanation: Ksp is the Solubility Product Constant and is the equilibrium constant that happens when a solid is dissolved in an aqueous solution.
The dissolution of chromium (III) hydroxide:
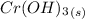 ⇄
⇄
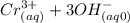
Every equilibrium constant is of the form:
![K = ([products]^(coefficient))/([reagents]^(coefficient))](https://img.qammunity.org/2021/formulas/chemistry/college/fx3cf9ewzcdl5glj20xr7borspq1c408fb.png)
Then,
![K_(sp) = [Cr^(3+)_((aq))][OH^(-)_(aq)]^(3)](https://img.qammunity.org/2021/formulas/chemistry/college/af9025br3x72i0913t5z7izqzuzsw1dthz.png)
The reagent is not included because solids don't take part in euqilibrium constants.
So, Ksp of chromium (III) hydroxide is
![K_(sp) = [Cr^(3+)_((aq))][OH^(-)_(aq)]^(3)](https://img.qammunity.org/2021/formulas/chemistry/college/af9025br3x72i0913t5z7izqzuzsw1dthz.png)