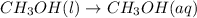Answer:
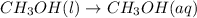
Step-by-step explanation:
According to the concept of “Like dissolves like”, the polar solutes dissolve in polar solvents, and non-polar solutes dissolve in non-polar solvents.
Covalent compounds which are formed by sharing of electrons between non metals, when dissolved in water, forms hydrogen bonding with water if they contain a electronegative atom along with hydrogen. Example: methanol
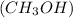
Ionic compounds which are made up of cations and anions, when dissolved in water, dissociate into ions. Example:
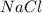
Thus equation which best represents methanol being dissolved in water is: