Answer:
d. To the left because Q > K_p
Step-by-step explanation:
Hello,
In this case, for the given reaction:
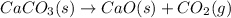
The pressure-based equilibrium expression is:
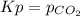
In such a way, since Kp is given we rather compute the reaction quotient at the specificed pressure of carbon dioxide as shown below:
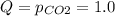
Therefore, since Q>Kp we can see that there are more products than reactants, which means that the reaction must shift leftwards towards the reactants in order to reestablish equilibrium, thus, answer is d. To the left because Q > Kp.
Regards.