Answer:
2.90
Step-by-step explanation:
Any buffer system can be described with the reaction:
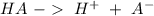
Where
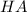 is the acid and
is the acid and
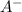 is the base. Additionally, the calculation of the pH of any buffer system can be made with the Henderson-Hasselbach equation:
is the base. Additionally, the calculation of the pH of any buffer system can be made with the Henderson-Hasselbach equation:
![pH=pKa~+~Log([A^-])/([HA])](https://img.qammunity.org/2021/formulas/chemistry/college/tijniqx72xtek8vbtq5yjl3lu4ewlq7dhw.png)
With all this in mind, we can write the reaction for our buffer system:
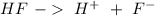
In this case, the acid is
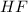 with a concentration of 0.413 M and the base is
with a concentration of 0.413 M and the base is
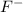 with a concentration of 0.237 M. We can calculate the pKa value if we do the "-Log Ka", so:
with a concentration of 0.237 M. We can calculate the pKa value if we do the "-Log Ka", so:
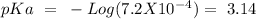
Now, we can plug the values into the Henderson-Hasselbach
![pH=~3.14~+~Log(([0.237~M])/([0.413~M]))~=~2.90](https://img.qammunity.org/2021/formulas/chemistry/college/3tcqiitnkzr2uv6frxsilsj6ocbul07t50.png)
The pH value would be 2.90
I hope it helps!