Answer:
Option a: positron emission.
Step-by-step explanation:
In the transformation we have:
⁶⁷Ga → ⁶⁷Zn
The reaction is:
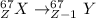
For Ga to become Zn, the atom nucleus has to lose a proton, so in the given options, the reaction that involves the transformation of a proton is the option a, positron emission.
In a positron emission, a proton becomes into a neutron and a positron:
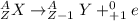
Therefore, the correct answer is option a: positron emission.
I hope it helps you!