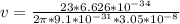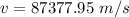Answer:
a

b
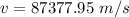
Step-by-step explanation:
From the question we are told that
The diameter is
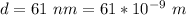
Generally the radius electron orbit is mathematically represented as
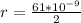
=>
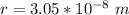
This radius can also be represented mathematically as
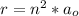
Here n is the quantum number and
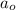 is the Bohr radius with a value
is the Bohr radius with a value
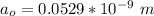
So
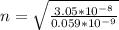
=>

Generally the angular momentum of the electron is mathematically represented as
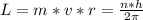
Here h is the Planck constant and the value is

m is the mass of the electron with values
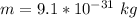
So