Answer:
Yes, the reaction is supported by spectrochemical series.
Step-by-step explanation:
When copper oxidizes with concentrated nitric acid, it produces copper ions; and the nitric acid is then reduced to nitrogen dioxide, a very poisonous gas which is brown in color and with an irritating odor.
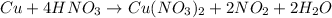
The copper ions (
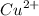 ) product which is initially coordinated to form nitrate ions from nitric acid and thus first gives the solution a green color.
) product which is initially coordinated to form nitrate ions from nitric acid and thus first gives the solution a green color.
Now when the solution is further diluted with water, the water molecules then displaces the nitrate ions around the copper ions and causes the solution to change its color to blue. The reaction is -
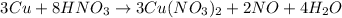
Yes, the results are supported by spectrochemical series.