Step-by-step explanation:
The De-Broglie wavelength in terms of potential difference is given by:
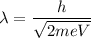
Where,
h is Planck's constant
m is mass of charged particle
V is potential difference
e is the amount of charge
It means that the De-Broglie wavelength is inversely proportional to the mass.
Since, the mass of the proton is more than the mass of the electron. So, the De- Broglie wavelength of the electron is larger than proton.