Answer:
A)
1. Reaction will shift rightwards towards the products.
2. It will turn green.
3. The solution will be cooler..
B) It will turn green.
Step-by-step explanation:
Hello,
In this case, for the stated equilibrium:
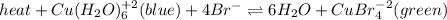
In such a way, by thinking out the Le Chatelier's principle, we can answer to each question:
A)
1. If potassium bromide, which adds bromide ions, is added more reactant is being added to the solution, therefore, the reaction will shift rightwards towards the products.
2. The formation of the green complex is favored, therefore, it will turn green.
3. The solution will be cooler as heat is converted into "cold" in order to reestablish equilibrium.
B) In this case, as the heat is a reactant, if more heat is added, more products will be formed, which implies that it will turn green.
Regards.