Answer:
Nitrogen 13
Step-by-step explanation:
We need fill in the blank the nuclide symbol for the missing particle in the following nuclear equation.
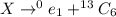
In this reaction, the emission of positron takes place. The atomic number in the LHS and the RHS should be same. So,
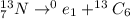
So, the LHS have nitrogen 13, a radioactive isotope.