Answer:
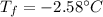
Step-by-step explanation:
Hello,
In this case, we can compute the the freezing point depression by using the following formula:
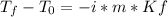
Whereas the freezing point of pure water is 0 °C van't Hoff factor for glucose is 1, the molality is computed as shown below and the freezing point constant of water is 1.86 °C/m:
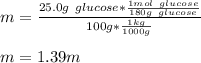
Thus, the freezing point of the solution is:
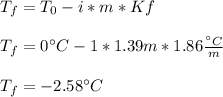
Regards.