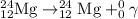Answer: a)
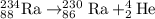
b)
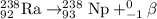
c)
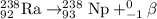
d)
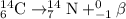
e)
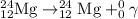
Step-by-step explanation:
A balanced nuclear equation is one in which the atomic number and mass number remains same on both sides of the equation i.e the number of protons and neutrons remain same.
General representation of an element is given as:
Z represents Atomic number
A represents Mass number
X represents the symbol of an element
a)
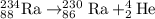
b)
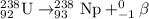
c)
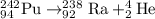
d)
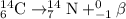
e)