Answer: The mole fraction of benzene will be 0.34 and mole fraction of toluene is 0.66
Step-by-step explanation:
According to Raoult's law, the vapor pressure of a component at a given temperature is equal to the mole fraction of that component multiplied by the vapor pressure of that component in the pure state.
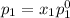 and
and
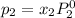
where, x = mole fraction in solution
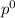 = pressure in the pure state
= pressure in the pure state
According to Dalton's law, the total pressure is the sum of individual pressures.
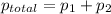
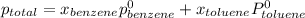
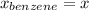 ,
,
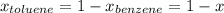 ,
,
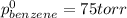
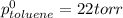
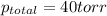
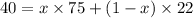
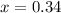
Thus (1-x0 = (1-0.34)=0.66
Thus the mole fraction of benzene will be 0.34 and that of toluene is 0.66