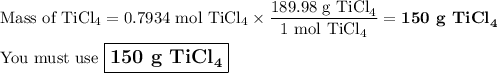Answer:
\large \boxed{\text{150 g TiCl}_{4}}
Step-by-step explanation:
We will need a balanced chemical equation with masses and molar masses, so, let's gather all the information in one place.
Mᵣ: 189.68 79.87
TiCl₄ + 2H₂O ⟶ TiO₂ + 4HCl
m/g: 50.0
To solve this stoichiometry problem, you must
- Convert the actual yield to the theoretical yield
- Use the molar mass of TiO₂ to convert the theoretical yield of TiO₂ to moles of TiO₂
- Use the molar ratio to convert moles of TiO₂ to moles of TiCl₄
- Use the molar mass of TiCl₄ to convert moles of TiCl₄ to mass of TiCt₄
1. Theoretical yield of TiO₂
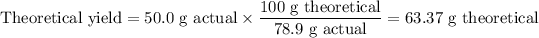
2. Moles of TiO₂
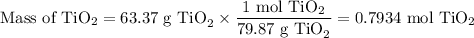
3, Moles of TiCl₄
The molar ratio is 1 mol TiO₂:1 mol TiCl₄.
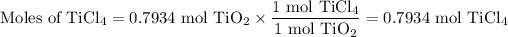
4. Mass of TiCl₄