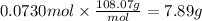Answer:
7.89 g
Step-by-step explanation:
Step 1: Write the balanced equation
S₈ + 16 F₂(g) → 8 SF₄
Step 2: Calculate the moles corresponding to 2.34 g of S₈
The molar mass of S₈ is 256.52 g/mol.
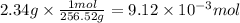
Step 3: Calculate the moles of SF₄ produced from 9.12 × 10⁻³ mol of S₈
The molar ratio of S₈ to SF₄ is 1:8. The moles of SF₄ produced are 8/1 × 9.12 × 10⁻³ mol = 0.0730 mol
Step 4: Calculate the mass corresponding to 0.0730 moles of SF₄
The molar mass of SF₄ is 108.07 g/mol.