Answer:
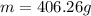
Step-by-step explanation:
Hello,
In this case, since the density is defined as the degree of compactness of a substance and it mathematically defined as the ratio of the mass and volume:
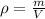
By knowing that its density is 1.11 g/mL, the mass in 366 mL is:
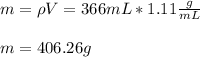
Regards.