Answer:
Solution ( for fourth attachment ) : 38°C
Tip : Remember the units °C when submitting answer
Step-by-step explanation:
As you mentioned, we only need the solution for the fourth attachment.
The idea here is that the heat lost by the metal will be equal to the heat gained by the water. We know that the specific heat gained or lost will always be represented by the following formula,
q = m
 c
c
Therefore if we substitute the know values and equate the two equations knowing that " q " is common among them --- ( 1 )
0.33
 448
448
Remember that the change in temperature of iron (ΔT) would be represented by final temperature - initial temperature, or final temperature - 693. Similarly the change in temperature of water will be final temperature - 39. Now we can pose the final temperature as a, and solve for a through substitution --- ( 2 )
0.33
 448
448
From here on take a look at the attachment. It represents how to receive get a through simple algebra. Here a, the final temperature, is about 38°C. In exact terms it will be
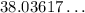 °C.
°C.