Answer:
The mass percentage of iron in
![\rm K_3[Fe(CN)_6]](https://img.qammunity.org/2021/formulas/chemistry/high-school/csbn06phzxi5yekz0vo544nkuu2y9xit6f.png) (potassium ferricyanide) is approximately
(potassium ferricyanide) is approximately
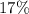 .
.
Step-by-step explanation:
Start by finding the formula mass of potassium ferricyanide,
![\rm K_3[Fe(CN)_6]](https://img.qammunity.org/2021/formulas/chemistry/high-school/csbn06phzxi5yekz0vo544nkuu2y9xit6f.png) , using relative atomic mass data from the question:
, using relative atomic mass data from the question:
![\begin{aligned} & M(\mathrm{K_3[Fe(CN)_6]}) \\ &\approx 3 * 39 + 56 + 6 * (12 + 14) \\ &= \rm 329 \; g \cdot mol^(-1)\end{aligned}](https://img.qammunity.org/2021/formulas/chemistry/high-school/vccednv14mlftfd8ias5hac6xzag2imjaa.png) .
.
In other words, every one mole of
![\rm K_3[Fe(CN)_6]](https://img.qammunity.org/2021/formulas/chemistry/high-school/csbn06phzxi5yekz0vo544nkuu2y9xit6f.png) formula units have a mass of approximately
formula units have a mass of approximately
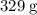 .
.
On the other hand, note that there is one iron
 atom in every one mole of
atom in every one mole of
![\rm K_3[Fe(CN)_6]](https://img.qammunity.org/2021/formulas/chemistry/high-school/csbn06phzxi5yekz0vo544nkuu2y9xit6f.png) formula units. Hence, there would be exactly one mole of iron atoms in every one mole of
formula units. Hence, there would be exactly one mole of iron atoms in every one mole of
![\rm K_3[Fe(CN)_6]](https://img.qammunity.org/2021/formulas/chemistry/high-school/csbn06phzxi5yekz0vo544nkuu2y9xit6f.png) formula units. The mass of that one mole of iron atoms is approximately
formula units. The mass of that one mole of iron atoms is approximately
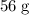 (again, from the relative atomic mass data of the question.)
(again, from the relative atomic mass data of the question.)
Therefore:
![\begin{aligned}& \text{Mass percentage of $\mathrm{Fe}$ in $\mathrm{K_3[Fe(CN)_6]}$} \\ &= \frac{\text{Mass of $\mathrm{Fe}$ in one mole of $\mathrm{K_3[Fe(CN)_6]}$ formula units}}{\text{Mass of one mole of $\mathrm{K_3[Fe(CN)_6]}$ formula units}}* 100\% \\ &= \frac{M(\mathrm{Fe}) * (\text{Number of $\mathrm{Fe}$ atoms in each $\mathrm{K_3[Fe(CN)_6]}$ formula unit)}}{M(\mathrm{K_3[Fe(CN)_6]})} * 100\%\end{aligned}](https://img.qammunity.org/2021/formulas/chemistry/high-school/dan2kjvdkvuqdegohhv02u4sb9u8axz7eh.png)
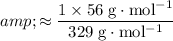 .
.